Company News

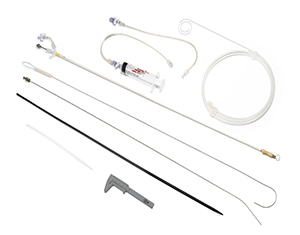
- SYM Medical obtained US FDA for featured product, Percutaneous balloon compression(PBC) kit[ 2020-11-10 ]SYM Medical obtained US FDA for featured product, Percutaneous balloon compression(PBC) kit.

- SYM Obtained MDSAP Approval[ 2019-04-10 ]SYM Obtained MDSAP Approval on 9th April of 2019 which was audited by BSI.
MDSAP was a new set of audit program co-sponsored by members of the IMDRF(International Medical Device Regulatory Forum) and recognized by regulators in the United States (FDA), Australia (TGA), Brazil (ANVISA), Canada (HC) and Japan (MHLW).
The program aims to establish a single audit process and audit requirements to meet and unity of these countries, which ensure the audit more comprehensive and effective.

- CCC is appreciated in top level Chinese Hospitals[ 2018-09-16 ]The Third People's Hospital of Huizhou is one of the top hospitals in Guangdong province, which specialized in cardiology, neurology and urology. There are 56 professional departments and 13 med-technical departments in the hospital; its total land area is nearly 109,000 square meters.

- SYM Passed BSI Unannounced Audit[ 2018-09-03 ]SYM Passed BSI Unannounced Audit
- SYM Obtained Algeria Import License[ 2018-07-03 ]SYM Obtained Algeria Import License





