Company News

- 2016 quality management system certificate (certificate code: MD 610101) issued by the British Standards Institution.[ 2018-02-27 ]2016 quality management system certificate (certificate code: MD 610101) issued by the British Standards Institution.

- SYM Cerebral Corridor Creator Set Obtained GDFDA Approval [ 2018-01-15 ]SYM’s Cerebral Corridor Creator Set has obtained the approval of Guangdong Food and Drug Administration (GDFDA) and received a Registration Certificate for Medical Device of People’s Republic of China, which is valid for five years.

- SYM’s PBMV balloon catheter has obtained approval from Central Drugs Standard Control Organization(CDSCO)[ 2017-12-27 ]SYM’s PBMV balloon catheter has obtained approval from Central Drugs Standard Control Organization(CDSCO)
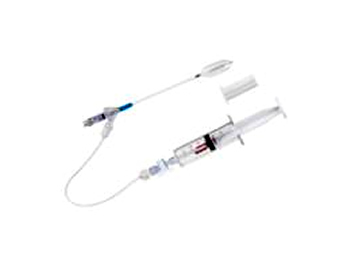
- Patent Granted for Cerebral Corridor Creator by SIPO[ 2016-04-13 ]The ground-breaking product of SYM, Cerebral Corridor Creator Set (CCC) was granted a utility model patent by State Intellectual Property Office (SIPO) of People’s Republic of China.

- SYM Opened its New Site[ 2016-04-12 ]On April 12th, 2016 SYM held the opening ceremony for its new site in Meisheng-Chuanggu Technology Park, Shenzhen. SYM Chairman and CEO Guan Guofeng and Vice CEO Wang Yuhui unveiled the new office. Over twenty guests from healthcare industry, clients, partners and SYM management attended the ceremony.





